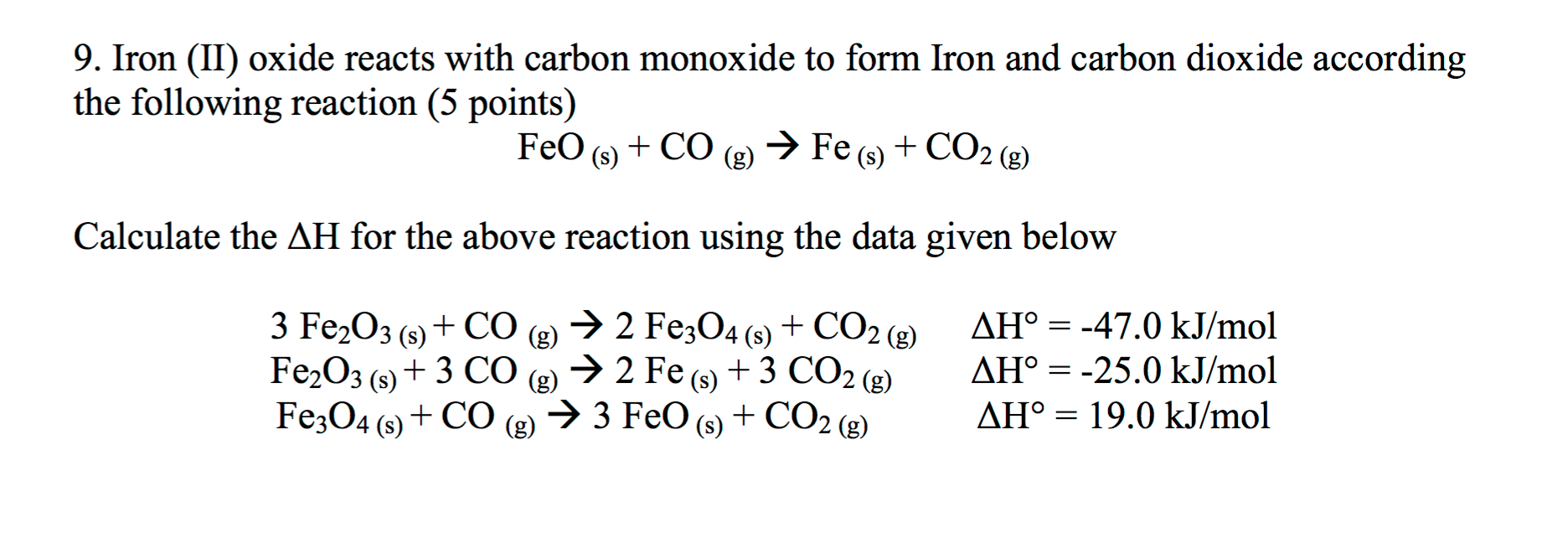
Iron Ii Oxide + Carbon Dioxide . to balance fe2o3 + c = fe + co2 you'll need to be sure to count all of atoms on each side of the chemical. G) magnesium carbonate solution plus aqueous hydrochloric acid. iron(iii) oxide react with carbon monoxide to produce iron(ii,iii) oxide and carbon dioxide. Synthetically, iron oxalate is heated to create iron (ii) oxide, carbon. in nature, it is formed from the incomplete oxidation (usually rusting) of iron (iii). f) solid iron (iii) oxide and carbon monoxide gas yields iron metal and carbon dioxide gas. to balance fe2o3 + co = fe + co2 you'll need to watch out for two things.
from www.chegg.com
Synthetically, iron oxalate is heated to create iron (ii) oxide, carbon. f) solid iron (iii) oxide and carbon monoxide gas yields iron metal and carbon dioxide gas. to balance fe2o3 + c = fe + co2 you'll need to be sure to count all of atoms on each side of the chemical. in nature, it is formed from the incomplete oxidation (usually rusting) of iron (iii). iron(iii) oxide react with carbon monoxide to produce iron(ii,iii) oxide and carbon dioxide. to balance fe2o3 + co = fe + co2 you'll need to watch out for two things. G) magnesium carbonate solution plus aqueous hydrochloric acid.
Solved Iron (II) Oxide Reacts With Carbon Monoxide To For...
Iron Ii Oxide + Carbon Dioxide to balance fe2o3 + c = fe + co2 you'll need to be sure to count all of atoms on each side of the chemical. G) magnesium carbonate solution plus aqueous hydrochloric acid. to balance fe2o3 + co = fe + co2 you'll need to watch out for two things. in nature, it is formed from the incomplete oxidation (usually rusting) of iron (iii). to balance fe2o3 + c = fe + co2 you'll need to be sure to count all of atoms on each side of the chemical. iron(iii) oxide react with carbon monoxide to produce iron(ii,iii) oxide and carbon dioxide. f) solid iron (iii) oxide and carbon monoxide gas yields iron metal and carbon dioxide gas. Synthetically, iron oxalate is heated to create iron (ii) oxide, carbon.
From www.researchgate.net
(PDF) Thermodynamic Study of Energy Consumption and Carbon Dioxide Iron Ii Oxide + Carbon Dioxide in nature, it is formed from the incomplete oxidation (usually rusting) of iron (iii). to balance fe2o3 + co = fe + co2 you'll need to watch out for two things. f) solid iron (iii) oxide and carbon monoxide gas yields iron metal and carbon dioxide gas. Synthetically, iron oxalate is heated to create iron (ii) oxide,. Iron Ii Oxide + Carbon Dioxide.
From www.chegg.com
Solved The reaction between iron(II) oxide and carbon Iron Ii Oxide + Carbon Dioxide f) solid iron (iii) oxide and carbon monoxide gas yields iron metal and carbon dioxide gas. Synthetically, iron oxalate is heated to create iron (ii) oxide, carbon. to balance fe2o3 + c = fe + co2 you'll need to be sure to count all of atoms on each side of the chemical. iron(iii) oxide react with carbon. Iron Ii Oxide + Carbon Dioxide.
From www.fishersci.se
Iron(II) oxide, 99.5 (metals basis), Alfa Aesar™ Other Iron Ii Oxide + Carbon Dioxide f) solid iron (iii) oxide and carbon monoxide gas yields iron metal and carbon dioxide gas. to balance fe2o3 + co = fe + co2 you'll need to watch out for two things. iron(iii) oxide react with carbon monoxide to produce iron(ii,iii) oxide and carbon dioxide. Synthetically, iron oxalate is heated to create iron (ii) oxide, carbon.. Iron Ii Oxide + Carbon Dioxide.
From www.chegg.com
Solved The reaction between iron (II) oxide and carbon Iron Ii Oxide + Carbon Dioxide to balance fe2o3 + c = fe + co2 you'll need to be sure to count all of atoms on each side of the chemical. iron(iii) oxide react with carbon monoxide to produce iron(ii,iii) oxide and carbon dioxide. f) solid iron (iii) oxide and carbon monoxide gas yields iron metal and carbon dioxide gas. G) magnesium carbonate. Iron Ii Oxide + Carbon Dioxide.
From www.slideserve.com
PPT Blast Furnace Reactions PowerPoint Presentation, free download Iron Ii Oxide + Carbon Dioxide Synthetically, iron oxalate is heated to create iron (ii) oxide, carbon. in nature, it is formed from the incomplete oxidation (usually rusting) of iron (iii). G) magnesium carbonate solution plus aqueous hydrochloric acid. f) solid iron (iii) oxide and carbon monoxide gas yields iron metal and carbon dioxide gas. iron(iii) oxide react with carbon monoxide to produce. Iron Ii Oxide + Carbon Dioxide.
From testbook.com
Iron (II) Oxide Formula Structure, Preparation & Properties Iron Ii Oxide + Carbon Dioxide G) magnesium carbonate solution plus aqueous hydrochloric acid. in nature, it is formed from the incomplete oxidation (usually rusting) of iron (iii). Synthetically, iron oxalate is heated to create iron (ii) oxide, carbon. to balance fe2o3 + co = fe + co2 you'll need to watch out for two things. iron(iii) oxide react with carbon monoxide to. Iron Ii Oxide + Carbon Dioxide.
From www.jaincolab.com
Iron (II) Oxide Manufacturer, Supplier & Exporter in India, Nigeria Iron Ii Oxide + Carbon Dioxide to balance fe2o3 + co = fe + co2 you'll need to watch out for two things. f) solid iron (iii) oxide and carbon monoxide gas yields iron metal and carbon dioxide gas. iron(iii) oxide react with carbon monoxide to produce iron(ii,iii) oxide and carbon dioxide. to balance fe2o3 + c = fe + co2 you'll. Iron Ii Oxide + Carbon Dioxide.
From www.nanochemazone.com
Iron(II) Oxide Powder Low Price 1 Nanochemazone Iron Ii Oxide + Carbon Dioxide iron(iii) oxide react with carbon monoxide to produce iron(ii,iii) oxide and carbon dioxide. to balance fe2o3 + co = fe + co2 you'll need to watch out for two things. Synthetically, iron oxalate is heated to create iron (ii) oxide, carbon. in nature, it is formed from the incomplete oxidation (usually rusting) of iron (iii). G) magnesium. Iron Ii Oxide + Carbon Dioxide.
From oneclass.com
OneClass The reaction between iron(II) oxide and carbon monoxide Iron Ii Oxide + Carbon Dioxide to balance fe2o3 + co = fe + co2 you'll need to watch out for two things. in nature, it is formed from the incomplete oxidation (usually rusting) of iron (iii). to balance fe2o3 + c = fe + co2 you'll need to be sure to count all of atoms on each side of the chemical. . Iron Ii Oxide + Carbon Dioxide.
From en.wikipedia.org
Iron(II) oxide Wikipedia Iron Ii Oxide + Carbon Dioxide to balance fe2o3 + co = fe + co2 you'll need to watch out for two things. in nature, it is formed from the incomplete oxidation (usually rusting) of iron (iii). G) magnesium carbonate solution plus aqueous hydrochloric acid. Synthetically, iron oxalate is heated to create iron (ii) oxide, carbon. f) solid iron (iii) oxide and carbon. Iron Ii Oxide + Carbon Dioxide.
From dxoymudcs.blob.core.windows.net
Iron Ii Oxide Equation at Stella Anderson blog Iron Ii Oxide + Carbon Dioxide to balance fe2o3 + co = fe + co2 you'll need to watch out for two things. G) magnesium carbonate solution plus aqueous hydrochloric acid. to balance fe2o3 + c = fe + co2 you'll need to be sure to count all of atoms on each side of the chemical. iron(iii) oxide react with carbon monoxide to. Iron Ii Oxide + Carbon Dioxide.
From www.showme.com
ShowMe iron Iron Ii Oxide + Carbon Dioxide to balance fe2o3 + co = fe + co2 you'll need to watch out for two things. f) solid iron (iii) oxide and carbon monoxide gas yields iron metal and carbon dioxide gas. iron(iii) oxide react with carbon monoxide to produce iron(ii,iii) oxide and carbon dioxide. in nature, it is formed from the incomplete oxidation (usually. Iron Ii Oxide + Carbon Dioxide.
From www.researchgate.net
Conceptual model of the effect of iron oxides on CO 2 emissions in Iron Ii Oxide + Carbon Dioxide Synthetically, iron oxalate is heated to create iron (ii) oxide, carbon. to balance fe2o3 + co = fe + co2 you'll need to watch out for two things. G) magnesium carbonate solution plus aqueous hydrochloric acid. in nature, it is formed from the incomplete oxidation (usually rusting) of iron (iii). f) solid iron (iii) oxide and carbon. Iron Ii Oxide + Carbon Dioxide.
From www.chegg.com
Solved ? iron (III) oxide + ? carbon monoxide iron (II) Iron Ii Oxide + Carbon Dioxide to balance fe2o3 + c = fe + co2 you'll need to be sure to count all of atoms on each side of the chemical. G) magnesium carbonate solution plus aqueous hydrochloric acid. iron(iii) oxide react with carbon monoxide to produce iron(ii,iii) oxide and carbon dioxide. in nature, it is formed from the incomplete oxidation (usually rusting). Iron Ii Oxide + Carbon Dioxide.
From www.numerade.com
At a certain temperature, iron (II) oxide, FeO, can react with carbon Iron Ii Oxide + Carbon Dioxide iron(iii) oxide react with carbon monoxide to produce iron(ii,iii) oxide and carbon dioxide. to balance fe2o3 + c = fe + co2 you'll need to be sure to count all of atoms on each side of the chemical. f) solid iron (iii) oxide and carbon monoxide gas yields iron metal and carbon dioxide gas. to balance. Iron Ii Oxide + Carbon Dioxide.
From www.chegg.com
Solved 1. The reaction between iron (II) oxide and carbon Iron Ii Oxide + Carbon Dioxide in nature, it is formed from the incomplete oxidation (usually rusting) of iron (iii). to balance fe2o3 + c = fe + co2 you'll need to be sure to count all of atoms on each side of the chemical. f) solid iron (iii) oxide and carbon monoxide gas yields iron metal and carbon dioxide gas. iron(iii). Iron Ii Oxide + Carbon Dioxide.
From www.chegg.com
Solved The reaction between iron(II) oxide and carbon Iron Ii Oxide + Carbon Dioxide G) magnesium carbonate solution plus aqueous hydrochloric acid. iron(iii) oxide react with carbon monoxide to produce iron(ii,iii) oxide and carbon dioxide. Synthetically, iron oxalate is heated to create iron (ii) oxide, carbon. to balance fe2o3 + c = fe + co2 you'll need to be sure to count all of atoms on each side of the chemical. . Iron Ii Oxide + Carbon Dioxide.
From www.numerade.com
SOLVED Iron (III) oxide reacts with carbon monoxide to produce iron Iron Ii Oxide + Carbon Dioxide to balance fe2o3 + co = fe + co2 you'll need to watch out for two things. in nature, it is formed from the incomplete oxidation (usually rusting) of iron (iii). Synthetically, iron oxalate is heated to create iron (ii) oxide, carbon. f) solid iron (iii) oxide and carbon monoxide gas yields iron metal and carbon dioxide. Iron Ii Oxide + Carbon Dioxide.