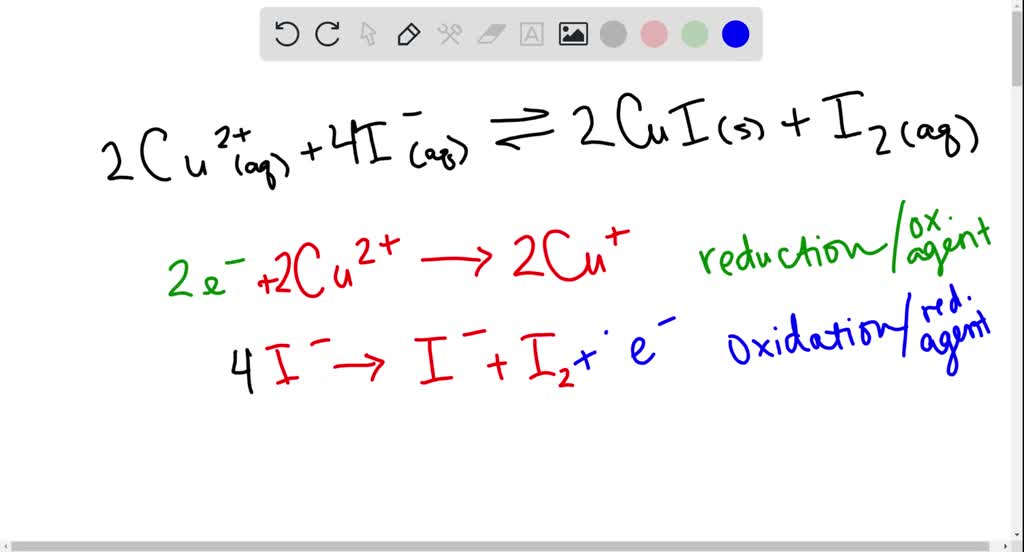
Copper Iodide Chemical Reaction . The reaction can be represented by the following. An example of using potassium iodide and copper(i) sulphate. Copper (ii) iodide (cui 2) is a chemical compound formed by copper in its +2 oxidation state and iodine. Copper(ii) ions oxidize iodide ions to molecular iodine, and in the process are themselves reduced to copper(i) iodide. Copper(ii) ions oxidise iodide ions to iodine, and in the process are themselves reduced to. However, it is less stable compared to. The reaction of hexaaquacopper(ii) ions with iodide ions. In the laboratory, copper (i) iodide is prepared by simply mixing an aqueous solutions of sodium or potassium iodide and a soluble copper (ii) salt. Do you want to learn how to write molecular, complete ionic, and net ionic equations for. Copper iodide can be prepared in a laboratory by a straightforward reaction of iodine with copper. Copper(i) iodide is usually made by the reduction of copper(ii) salts.
from www.numerade.com
The reaction of hexaaquacopper(ii) ions with iodide ions. Do you want to learn how to write molecular, complete ionic, and net ionic equations for. However, it is less stable compared to. The reaction can be represented by the following. Copper(ii) ions oxidise iodide ions to iodine, and in the process are themselves reduced to. Copper iodide can be prepared in a laboratory by a straightforward reaction of iodine with copper. Copper(ii) ions oxidize iodide ions to molecular iodine, and in the process are themselves reduced to copper(i) iodide. An example of using potassium iodide and copper(i) sulphate. In the laboratory, copper (i) iodide is prepared by simply mixing an aqueous solutions of sodium or potassium iodide and a soluble copper (ii) salt. Copper(i) iodide is usually made by the reduction of copper(ii) salts.
SOLVED Aqueous copper(II) ion reacts with aqueous iodide ion to yield
Copper Iodide Chemical Reaction The reaction of hexaaquacopper(ii) ions with iodide ions. Copper(i) iodide is usually made by the reduction of copper(ii) salts. In the laboratory, copper (i) iodide is prepared by simply mixing an aqueous solutions of sodium or potassium iodide and a soluble copper (ii) salt. An example of using potassium iodide and copper(i) sulphate. However, it is less stable compared to. Copper iodide can be prepared in a laboratory by a straightforward reaction of iodine with copper. Do you want to learn how to write molecular, complete ionic, and net ionic equations for. Copper(ii) ions oxidise iodide ions to iodine, and in the process are themselves reduced to. Copper(ii) ions oxidize iodide ions to molecular iodine, and in the process are themselves reduced to copper(i) iodide. The reaction of hexaaquacopper(ii) ions with iodide ions. The reaction can be represented by the following. Copper (ii) iodide (cui 2) is a chemical compound formed by copper in its +2 oxidation state and iodine.
From favpng.com
Copper(I) Iodide Magnesium Iodide Crystal Structure Molecule, PNG Copper Iodide Chemical Reaction In the laboratory, copper (i) iodide is prepared by simply mixing an aqueous solutions of sodium or potassium iodide and a soluble copper (ii) salt. Copper(ii) ions oxidise iodide ions to iodine, and in the process are themselves reduced to. The reaction of hexaaquacopper(ii) ions with iodide ions. However, it is less stable compared to. Do you want to learn. Copper Iodide Chemical Reaction.
From www.semanticscholar.org
Figure 1 from Selective copper(II) acetate and potassium iodide Copper Iodide Chemical Reaction The reaction can be represented by the following. The reaction of hexaaquacopper(ii) ions with iodide ions. An example of using potassium iodide and copper(i) sulphate. Copper iodide can be prepared in a laboratory by a straightforward reaction of iodine with copper. Copper(ii) ions oxidize iodide ions to molecular iodine, and in the process are themselves reduced to copper(i) iodide. However,. Copper Iodide Chemical Reaction.
From pubs.acs.org
Aryl Radical Enabled, CopperCatalyzed SonogashiraType CrossCoupling Copper Iodide Chemical Reaction The reaction can be represented by the following. In the laboratory, copper (i) iodide is prepared by simply mixing an aqueous solutions of sodium or potassium iodide and a soluble copper (ii) salt. Copper iodide can be prepared in a laboratory by a straightforward reaction of iodine with copper. However, it is less stable compared to. Copper(ii) ions oxidise iodide. Copper Iodide Chemical Reaction.
From askfilo.com
In the double displacement reaction between aqueous potassium iodide and Copper Iodide Chemical Reaction An example of using potassium iodide and copper(i) sulphate. The reaction can be represented by the following. Copper iodide can be prepared in a laboratory by a straightforward reaction of iodine with copper. Copper (ii) iodide (cui 2) is a chemical compound formed by copper in its +2 oxidation state and iodine. The reaction of hexaaquacopper(ii) ions with iodide ions.. Copper Iodide Chemical Reaction.
From www.youtube.com
Reaction of Copper sulfate (CuSO4) with Potassium iodide (KI) YouTube Copper Iodide Chemical Reaction An example of using potassium iodide and copper(i) sulphate. Copper(ii) ions oxidize iodide ions to molecular iodine, and in the process are themselves reduced to copper(i) iodide. In the laboratory, copper (i) iodide is prepared by simply mixing an aqueous solutions of sodium or potassium iodide and a soluble copper (ii) salt. Copper(i) iodide is usually made by the reduction. Copper Iodide Chemical Reaction.
From www.youtube.com
Copper Chemistry Cu(I)Iodide (Redox) YouTube Copper Iodide Chemical Reaction Copper(ii) ions oxidize iodide ions to molecular iodine, and in the process are themselves reduced to copper(i) iodide. Copper iodide can be prepared in a laboratory by a straightforward reaction of iodine with copper. Copper(i) iodide is usually made by the reduction of copper(ii) salts. Copper (ii) iodide (cui 2) is a chemical compound formed by copper in its +2. Copper Iodide Chemical Reaction.
From www.youtube.com
Empirical Formula of Copper Iodide Virtual Lab Student B YouTube Copper Iodide Chemical Reaction Copper iodide can be prepared in a laboratory by a straightforward reaction of iodine with copper. Copper(ii) ions oxidize iodide ions to molecular iodine, and in the process are themselves reduced to copper(i) iodide. An example of using potassium iodide and copper(i) sulphate. Copper (ii) iodide (cui 2) is a chemical compound formed by copper in its +2 oxidation state. Copper Iodide Chemical Reaction.
From www.semanticscholar.org
Copper Iodide and Copper (I) Oxide Based Hole Transport Materials for Copper Iodide Chemical Reaction An example of using potassium iodide and copper(i) sulphate. The reaction can be represented by the following. In the laboratory, copper (i) iodide is prepared by simply mixing an aqueous solutions of sodium or potassium iodide and a soluble copper (ii) salt. Copper iodide can be prepared in a laboratory by a straightforward reaction of iodine with copper. However, it. Copper Iodide Chemical Reaction.
From www.numerade.com
SOLVEDAqueous copper(II) ion reacts with aqueous iodide ion to yield Copper Iodide Chemical Reaction Copper(ii) ions oxidize iodide ions to molecular iodine, and in the process are themselves reduced to copper(i) iodide. An example of using potassium iodide and copper(i) sulphate. Copper(i) iodide is usually made by the reduction of copper(ii) salts. The reaction of hexaaquacopper(ii) ions with iodide ions. The reaction can be represented by the following. Copper(ii) ions oxidise iodide ions to. Copper Iodide Chemical Reaction.
From www.semanticscholar.org
Figure 3 from Curative Effects of Copper Iodide Embedded on Gallic Acid Copper Iodide Chemical Reaction Copper (ii) iodide (cui 2) is a chemical compound formed by copper in its +2 oxidation state and iodine. The reaction can be represented by the following. The reaction of hexaaquacopper(ii) ions with iodide ions. An example of using potassium iodide and copper(i) sulphate. In the laboratory, copper (i) iodide is prepared by simply mixing an aqueous solutions of sodium. Copper Iodide Chemical Reaction.
From www.youtube.com
How to Write the Formula for Copper (II) iodide YouTube Copper Iodide Chemical Reaction The reaction of hexaaquacopper(ii) ions with iodide ions. However, it is less stable compared to. Copper(ii) ions oxidize iodide ions to molecular iodine, and in the process are themselves reduced to copper(i) iodide. Copper iodide can be prepared in a laboratory by a straightforward reaction of iodine with copper. Do you want to learn how to write molecular, complete ionic,. Copper Iodide Chemical Reaction.
From www.fishersci.com
Copper(I) iodide, 98, Thermo Scientific Chemicals, Quantity 250g Copper Iodide Chemical Reaction Copper(i) iodide is usually made by the reduction of copper(ii) salts. Copper(ii) ions oxidise iodide ions to iodine, and in the process are themselves reduced to. Do you want to learn how to write molecular, complete ionic, and net ionic equations for. The reaction of hexaaquacopper(ii) ions with iodide ions. An example of using potassium iodide and copper(i) sulphate. Copper. Copper Iodide Chemical Reaction.
From www.numerade.com
SOLVED Aqueous copper(II) ion reacts with aqueous iodide ion to yield Copper Iodide Chemical Reaction Copper (ii) iodide (cui 2) is a chemical compound formed by copper in its +2 oxidation state and iodine. The reaction of hexaaquacopper(ii) ions with iodide ions. Copper(ii) ions oxidize iodide ions to molecular iodine, and in the process are themselves reduced to copper(i) iodide. Copper(ii) ions oxidise iodide ions to iodine, and in the process are themselves reduced to.. Copper Iodide Chemical Reaction.
From www.youtube.com
Copper Sulfate and Potassium Iodide Solutions YouTube Copper Iodide Chemical Reaction The reaction of hexaaquacopper(ii) ions with iodide ions. The reaction can be represented by the following. However, it is less stable compared to. An example of using potassium iodide and copper(i) sulphate. Copper(ii) ions oxidize iodide ions to molecular iodine, and in the process are themselves reduced to copper(i) iodide. Copper(i) iodide is usually made by the reduction of copper(ii). Copper Iodide Chemical Reaction.
From www.chegg.com
Solved The primary route for making copper iodide is by Copper Iodide Chemical Reaction In the laboratory, copper (i) iodide is prepared by simply mixing an aqueous solutions of sodium or potassium iodide and a soluble copper (ii) salt. However, it is less stable compared to. The reaction of hexaaquacopper(ii) ions with iodide ions. Copper(ii) ions oxidise iodide ions to iodine, and in the process are themselves reduced to. Copper(ii) ions oxidize iodide ions. Copper Iodide Chemical Reaction.
From pubs.acs.org
A Copper Iodide ClusterBased Coordination Polymer as an Unconventional Copper Iodide Chemical Reaction Copper(ii) ions oxidise iodide ions to iodine, and in the process are themselves reduced to. However, it is less stable compared to. Copper iodide can be prepared in a laboratory by a straightforward reaction of iodine with copper. The reaction can be represented by the following. Do you want to learn how to write molecular, complete ionic, and net ionic. Copper Iodide Chemical Reaction.
From www.scribd.com
Copper I Iodide PDF Chemical Reactions Cysteine Copper Iodide Chemical Reaction An example of using potassium iodide and copper(i) sulphate. Copper(ii) ions oxidise iodide ions to iodine, and in the process are themselves reduced to. However, it is less stable compared to. The reaction of hexaaquacopper(ii) ions with iodide ions. Copper(i) iodide is usually made by the reduction of copper(ii) salts. The reaction can be represented by the following. Do you. Copper Iodide Chemical Reaction.
From worksheetdbshiite.z19.web.core.windows.net
Precipitation Reaction Examples Chemistry Copper Iodide Chemical Reaction Copper iodide can be prepared in a laboratory by a straightforward reaction of iodine with copper. Copper(ii) ions oxidize iodide ions to molecular iodine, and in the process are themselves reduced to copper(i) iodide. The reaction of hexaaquacopper(ii) ions with iodide ions. However, it is less stable compared to. The reaction can be represented by the following. Do you want. Copper Iodide Chemical Reaction.